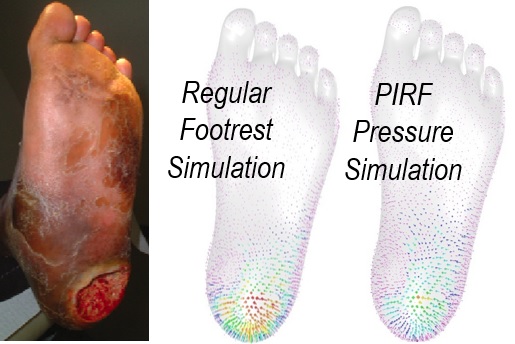
Kemper Engineering Services has engineers experienced in biology, medical, and biotech fields. Our principal engineer is a multi-tour combat veteran with trauma response training, has overseen hospital and clinic construction, and served on civilian medical missions prior to 9/11. We have several members of the ASME Codes and Standards Committee for Pressure Vessels for Human Occupancy, which includes medical hyperbaric chambers. Our team has presented papers and poster sessions involving engineering analysis and simulation of medical conditions such as pressure sores, which are can be modeled through Finite Element Analysis, and Mal de Debarquement Syndrom (MdDS), a motion-induced neurological condition. Our team of consultants include biomedical engineers, medical equipment consultants, and government policy experts.
One of our current projects is examining the standards for heating, ventilation, and air conditioning (HVAC) and whether they are sufficient to address the issues with disease such as COVID-19. We are working with ASHRAE members to examine the standards and compare the results to the evolving CDC guidance. This could have long-term effects on larger buildings and occupancy standards.
Our previous projects include:
- Developing a pressure injury reducing device, including IRB-approved human testing.
- Assisting in the development of medical devices for clients
- Examination of complex ergonomics, both external (overall body articulation) and internal (specific organs and muscle groups).
- Assisting in the development of patents and patent drawing for over a dozen medical device patents
- Assisting in the development of food-safe working equipment for clients
- Troubleshooting, design, and analysis of food processing equipment
- Assisting in the development of an automated USP-71 contamination testing system
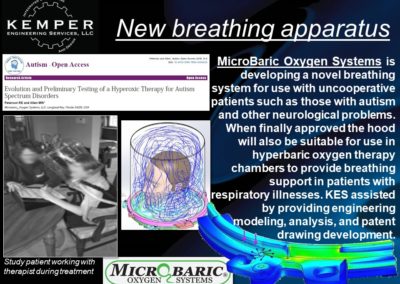
- Developing a novel life-support system
- Design and 3rd party independent review of medical hyperbaric equipment or facilities
- Review and analysis of a failed hyperbaric medical treatment chamber
- Review of medical files to assess whether observed injuries are consistent with the physics of the incident
- Investigating the correlation of engineering literature and results to medical literature to help link or debunk causation
- Predicting the human response to various mechanical loads and conditions
It is important to stress none of our full-time team are medical personnel. We work with the doctors, nurses, first responders, technicians, medical researchers, and other technical professionals who are subject matter experts. We work as part of a team to provide the expertise needed to resolve the challenges.
KES services for the medical, food, and biotech industries includes:
- Medical device review
- Food-safe equipment design
- Prototype development
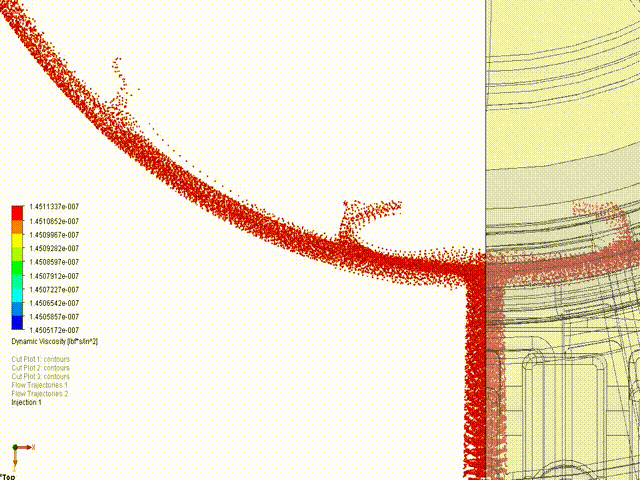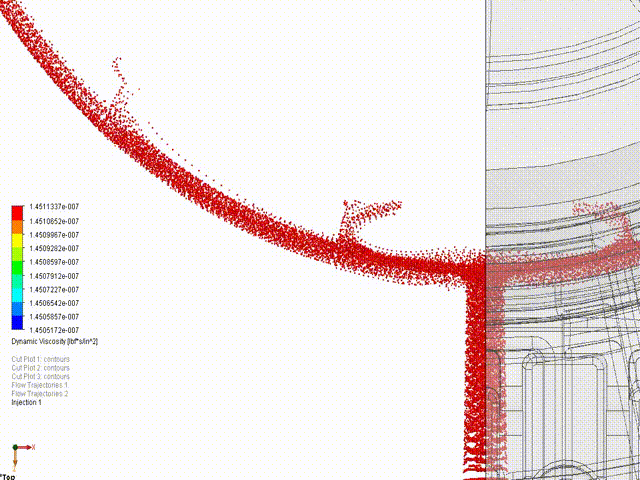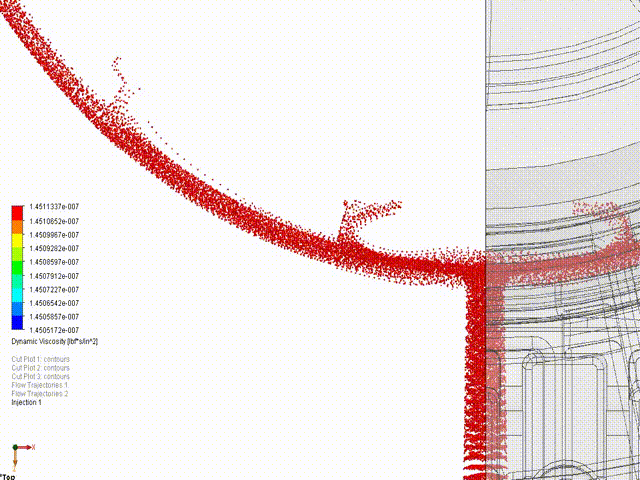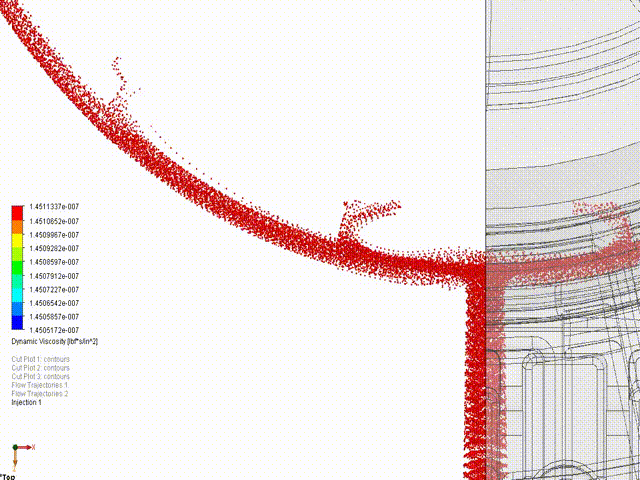
- Engineering simulations (Stress, loads, kinematics, fluid flow, gas mixing)
- Modeling human-equipment interactions, to include predicting conditions consistent with specific injury modes
- Ergonomic analysis and review (including 3D modeling)
- Failure Modes and Effects Analysis (FMEA)
- Design of Experiment (DoE)
- Patent and patent drawing development
- FDA 510(k) support
Hyperbaric Technology: KES has assisted many companies with established and novel designs for hyperbaric technology, ranging from high-pressure deep saturation diving support at 50+ atmospheres to fractional-atmosphere treatment hoods. ASME PVHO rules apply at pressures of 2 psig and higher for chamber and chamber component design. While there is debate amongst medical practitioners regarding “wellness” vs “medical” vs “occupational” treatments, our concern is ensuring the systems are safe, consistent with established engineering practice, and meet the appropriate jurisdictional requirements. In the US, current policy is that all pressure vessels for human occupancy fall under the FDA’s authority, while local installation must comply with the fire protection code. KES reserves the right to step away from any project in which we assess that the participants are not in compliance with the jurisdictional authorities. If we are aware of risks to the public, our licensed professionals are required by law to act, just as they are required to act in any engineering application.
Key Clients
- 510K FDA Consulting (Hialeah, FL)
- Allpax (Covington, LA)
- Bioceptive (New Orleans, LA)
- Blanson Acrylic Engineering (Brighton, England)
- Cocktail Kingdom (New York, NY)
- Equine Oxygen Therapy (Lexington, KY)
- Food + Beverage Innovation (Portland, OR)
- The Healing Sole (Baton Rouge, LA)
- HM Recompression (Maitland, FL)
- Macy-Pan Hyperbaric Systems (Shanghai, China)
- MicroBaric Oxygen Systems, LLC (Longboat Key, FL)
- Rapid Micro Biosystems (Lowell, MA)
- Reimers Systems, Inc. (Lorton, VA)
- Solvay USA, Inc. (Princeton, NJ)
- Tekni-Plex (Wayne, PA)
- Three Roll Distillery (Baton Rouge, LA)